Successful completion of development of a precision therapeutic system designed to safely achieve high concentration…
PRESS RELEASE: Advanced Chemotherapy Technologies, Inc. Files IND Application for First in Human use of ACT-IOP-003 in Pancreatic Cancer Patients
Raleigh, NC, March 20th, 2020 – Advanced Chemotherapy Technologies, Inc. (www.advancedchemotech.com), a combination drug-device company developing the ACT Implantable Iontophoresis Chemotherapy Delivery Device with gemcitabine (ACT-IOP-003), announced today that the Company has submitted an investigational new drug (IND) application to the U.S. Food and Drug Administration (FDA) to conduct a Phase 1 clinical trial with ACT-IOP-003 as a first in human safety trial for patients who are currently approved for surgical resection of their pancreatic cancer.
Every day, an estimated 150 Americans will be diagnosed with pancreatic cancer, and about 120 people will die from the disease. One of the major challenges of pancreatic cancer that contributes to its poor survival rates is the development of resistance to standard chemotherapy. Heterogeneity of the tumor, the dense fibroblastic stroma, and the aggressive biology of the tumor all contribute to chemoresistance. Furthermore, as the aggressive tumor grows into adjacent tissues, it can invade the liver or the stomach, and more often invades local vasculature, rendering the tumor inoperable. As surgical resection is currently the gold standard for treatment, a locally advanced tumor that is non-resectable leaves patients in a state where only palliative care may be offered.
Tony Voiers, CEO of ACT explains: “ACT has conducted extensive preclinical safety and efficacy testing, and this IND submission represents thousands of hours of detailed research, development and verification work, along with extensive clinical protocol development. We believe we are now well positioned to conduct a first-in-human study and we are proud to present all of our findings to the FDA”.
The original iontophoresis technology was developed at the UNC School of Medicine by collaborating scientists Joseph DeSimone, Ph.D., then M.D./Ph.D. student James Byrne and Jen Jen Yeh, M.D. DeSimone is a chancellor’s eminent professor of chemistry at UNC and the William R. Kenan Jr. Distinguished Professor of Chemical Engineering at N.C. State University and of Chemistry at UNC. DeSimone is also co-founder and CEO of Carbon3D. Byrne is now a Clinical Fellow in Radiation Oncology at Massachusetts General Hospital. Yeh is Professor and Vice Chair of Research, Surgery-Oncology and Pharmacology at UNC-Chapel Hill’s Lineberger Comprehensive Cancer Center. ACT holds exclusive rights to the patented technology.
About ACT
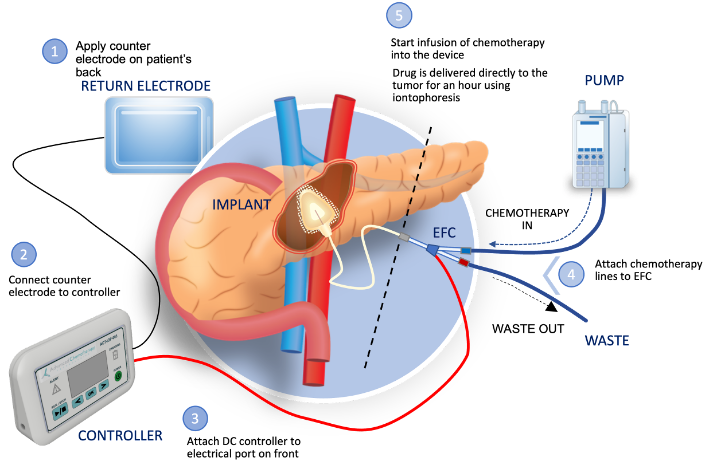
ACT is a biotechnology company developing implantable devices to infuse chemotherapy drugs directly into affected organs, targeting difficult-to-reach tumors while largely sparing surrounding tissues, organs and blood vessels. Our first device, about the size of a quarter, will be implanted in the pancreas with electrical leads running to the abdomen. The device uses a process called iontophoresis that drives the chemotherapy into the tumor using electrical currents that pass through the drug solution into the tissue. The desmoplastic stroma that is a barrier to systemic chemotherapy because of poor diffusion from the blood vessels can now be opened through a process known as reversible electroporation, permitting the chemotherapy to pass into the tumor. This approach for more precise drug delivery is designed to shrink tumors enough for surgeons to remove them.While not always curative, that improvement could extend those patients’ life expectancy. ACT is headquartered in Raleigh, NC. For more information about ACT, visit www.advancedchemotech.com or email [email protected].
SOURCE: Advanced Chemotherapy Technologies, Inc.
Released June 20, 2020