Ness Bermingham, Ph.D. Khosla Ventures partner and Intellia Therapeutics founder, joins Board. Advance of IOP…

Focal Medical Receives Award from NCI to Develop Treatment for Oral Cavity Cancer
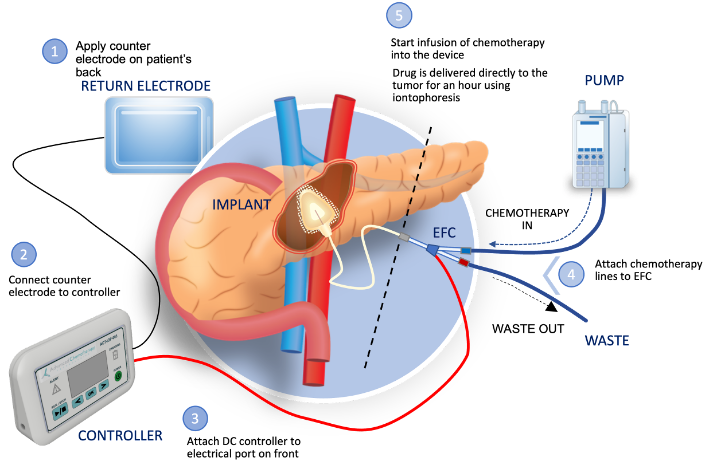
Cary, North Carolina — Focal Medical, Inc., (“Focal”) a privately held biopharmaceutical company developing a targeted therapeutic system to treat inoperable tumors and to deliver genomic medicines, today announced that it has received a $300,000 award from the National Cancer Institute (NCI) Small Business Innovation Research (SBIR) Innovative Concept Award Program. The award is to develop a product using Focal’s iontophoresis platform (IOP) to deliver platinum-based chemotherapy directly to the target tumor for the treatment of local recurrent oral cavity cancer.
The NCI program awards businesses developing highly innovative and transformative technologies that have the potential to create new scientific paradigms and establish entirely new and improved approaches to significantly improve research, prevention, detection, and care in pediatric and rare cancers. Rare cancers with a 5-year survival rate of less than 50% were particularly encouraged to apply.
Focal’s therapeutic product is designed to address the unmet medical need for the significant number of patients with oral cavity cancer who experience disease recurrence[1] following first line treatment with surgery, radiation, or chemotherapy. Patients with recurrent disease have few treatment options and a 30% 5-year survival rate[2].
In collaboration with Virginia Tech’s Animal Cancer Care and Research Center, Focal has evaluated its targeted therapeutic system’s ability to deliver platinum-based chemotherapy directly to naturally occurring oral cavity cancers in companion canines. This study has demonstrated that Focal’s targeted therapeutic system can deliver these drugs at high concentration directly and specifically to target tumor tissue with little to no systemic exposure. In addition, a single tumor-targeted treatment for 60 minutes resulted in a significant reduction in tumor volume as measured by CT scan in just 21 days post treatment. This study is ongoing and continues to enroll companion canines with oral cavity cancers. For more information on enrollment, visit https://research.vetmed.vt.edu/clinical-trials/current-studies/iontophoresis.html.
Focal Medical believes the targeted delivery of cisplatin directly to oral cavity tumors has the potential to improve outcomes for patients with recurrent disease by shrinking tumors prior to other treatment.
About Focal Medical
Focal Medical, Inc. is a privately held, biopharmaceutical company developing novel therapeutic products based on its innovative and patent protected targeted therapeutic system. The Company’s lead product is a targeted therapeutic product delivering gemcitabine (an FDA approved chemotherapeutic) actively and directly to the pancreas by non-circulatory pathways to treat pancreatic cancer. Focal Medical is expanding its product focus into therapies for other solid tumors and genomic medicine products. Its products utilize its innovative energy-based targeted therapeutic system. Focal Medical’s patented iontophoresis delivery system enables the internal, site-specific delivery of therapeutics actively, directly, and selectively to the target organ using non-circulatory pathways. The technology thus addresses certain significant challenges and limitations of traditional systemic drug delivery including systemic toxicity and barriers to therapeutic effect. Please visit our website at: www.focalmedical.co.
Company contacts:
Michael Aldridge, CEO
(919) 651-4656
[email protected]
Tony Voiers, COO
(919) 917-7324
[email protected]
Investor Contact:
Burns McClellan
Lee Roth / Eric Ando
[email protected] / [email protected]
[1]https://www.cancer.org/cancer/types/oral-cavity-and-oropharyngeal-cancer/about/key-statistics.html, Carvalho et. al, Oral Oncology, 2003, Shetty et. al, Frontiers in Oral Health, 2021, Zittel et. al, Clinical Oral Investigations, 2021
[2] Wang et. al, Chin J Cancer, 2013